The Gram Formula Mass of Nh4cl Is
What is the gram formula mass of CaOH_2. We convert the moles to mass in order to know how many grams of NH₄Cl are produced 0137 mol.

The Four Intermolecular Forces And How They Affect Boiling Points Intermolecular Force Chemistry Lessons Ap Chem
Correct answer is 1 - one mole of NO2.

. Gram formula mass can be defined as the mass of 1 mole 6022 x 10²³ particles of compound in grams. Molar mass of MgCl2 9521952112525076 mole1 mole would have 602x1023 formula units so 076 moles would have076 x. 9 moles NH4Cl to.
140067 1007944 35453 Percent composition by element. 5 moles NH4Cl to grams 2674573 grams. 1 Ca 40 gram 2 OH217 gram Sum these up 74 grams per mole.
Get control of 2021. 4 moles NH4Cl to grams 21396584 grams. 6 moles NH4Cl to grams 32094876 grams.
The answer must always be written with the unit gmol grams per mole. Ammonium chlorideA few things to consider when finding the molar mass for NH4Cl- make sure you have the. Explanation of how to find the molar mass of NH4Cl.
So 4 atoms 4u MASS OF CL -35u. This is similar to molar mass. Molar mass of NH4Cl.
1 Answer G_Ozdilek Aug 23 2016 Ca40 and 2OH34. The gram formula mass of NH4Cl is. 0436 moles of ammonium chloride NH4Cl has a mass of 233 grams.
This compound is also known as Ammonium Chloride. Therefore the mass in grams or gram formula mass 53 grams. A 940 g C 132 g B 114 g D 660 g.
How many gram of H is in 5921 x 10 -2 g of NH 4 Cl. Convert grams NH4Cl to moles or moles NH4Cl to grams. Formula mass of NH 4 Cl is 5350.
Click to see full answer. You can view more details on each measurement unit. 2 moles NH4Cl to grams 10698292 grams.
Gram formula mass is also calculated by adding the total mass of each element present in the given formula. The sum of the atomic masses of the atoms in one molecule of C3H6Br2 is called the. Molar mass of NH4Cl is 534915 gmol.
Up to 24 cash back 1. The gram formula mass of NH4Cl is 1 224 gmole 2 280 gmole 3 535 gmole 4 955 gmole. TOTAL MASS 4 14 35 53 u or 53 amu.
The sum of the atomic masses of the atoms in one molecule of C3H6Br2 is called the 1 formula mass 2 isotopic mass 3 percent abundance 4 percent composition. The gram formula mass of nh4cl is1 224 gmole 3 535 gmole2 280 gmole 4 955 gmole. Finding this answer involves using the number of atoms of each element in the molecular formula for this compound and their atomic masses.
3 moles NH4Cl to grams 16047438 grams. Molar mass of NH4Cl 5349146 gmol. Up to 256 cash back The gram formula mass of NH 4 Cl is 1 224 gmole 2 280 gmole 3 535 gmole 4 955 gmole.
Chemistry Matter Atomic Mass. The gram formula mass of NH4Cl is 1 224 gmole 3 535 gmole 2 280 gmole 4 955 gmole. The gram formula mass of a compound is 48 grams.
How many formula units of MgCl2 are found in 12525 grams of the compound. Molar mass is defined as the atomic mass of one mole of an element molecular compound or ionic compound. 1 mole is equal to 1 moles NH4Cl or grams.
7 moles NH4Cl to grams 37444022 grams. Then the answer is 74 grams per mol. The molar mass of NH4Cl ammonium chloride is.
The molar mass of NH43PO4 is 149087 grams per mole. The SI base unit for amount of substance is the mole. 1 moles NH4Cl to grams 5349146 grams.
8 moles NH4Cl to grams 42793168 grams. Molecular weight of NH4Cl or grams This compound is also known as Ammonium Chloride. Convert between NH4Cl weight and moles.
Calculate the mass in grams of solid ammonium chloride NH4Cl formula mass 535 gmole that must be added to 300 mL of 025 M NH3 solution to make a. How do we then get from moles to mass. Now in NH4CL MASS OF N - 14 u note u - units MASS OF H - 1 X 4 4 u note the mass of 1 atom is 1.
Mass moles molar mass mnM. Compound name is ammonium chloride. Track your food intake exercise sleep and meditation for free.
Gram formula mass aka. NH4Cl molecular weight. From the molecular formula NH43PO4 the number of atoms for each element is three atoms of nitrogen twelve atoms of hydrogen one atom of phosphorus.
See also our theoretical yield calculator for chemical reactions probably your next stop to finish the problem set. Ca 40 gram O 16 gram H1 gram OH17 gram. The percent composition by mass of nitrogen in NH4OH gram-formula mass 35 gramsmole is equal to.
What Is The Gram Formula Mass Of Nh4cl Is How Is It Calculated Quora

Calcium Hydroxide And Ammonium Chloride React To Give Ammonia As Per Equation Ca Oh 2 2 Nh 4 Cl To Cacl 2 2 Nh 3 2 H 2 O In A Reaction 5 35 G Of Ammonium

Ammonium Chloride Acs Grade 500 Grams Amazon Com Industrial Scientific

Math Of Chemistry Ppt Download

What Is The Molar Mass Of Ammonium Chloride Molecular Formula Youtube

How To Convert Moles Of Nh4cl To Grams Youtube
What Is The Gram Formula Mass Of Nh4cl Is How Is It Calculated Quora

Calculate The Mass Of Nh4cl That Must Be Completely Dissolved In 1 Litre Of Aqueous Solution To Attain An Osmotic Pressure Of 5 Atm At 298 K Assume Ideal Behaviour

Molar Mass Of Nh4cl Ammonium Chloride Youtube

Write The Balanced Equation For The Reaction Between A Mixture Of Ammonium Chloride And Slaked Lime
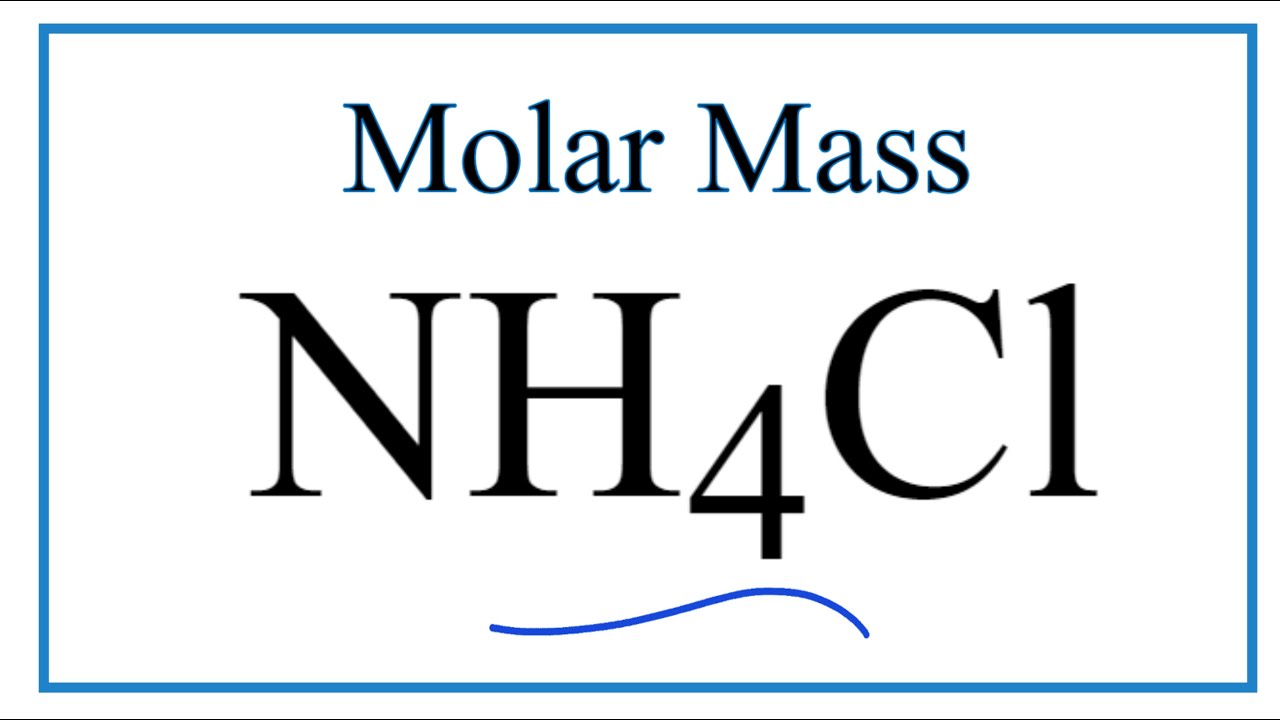
Molar Mass Molecular Weight Of Nh4cl Ammonium Chloride Youtube

Ammonium Chloride Nh4cl Mole To Weight Youtube
What Is The Gram Formula Mass Of Nh4cl Is How Is It Calculated Quora
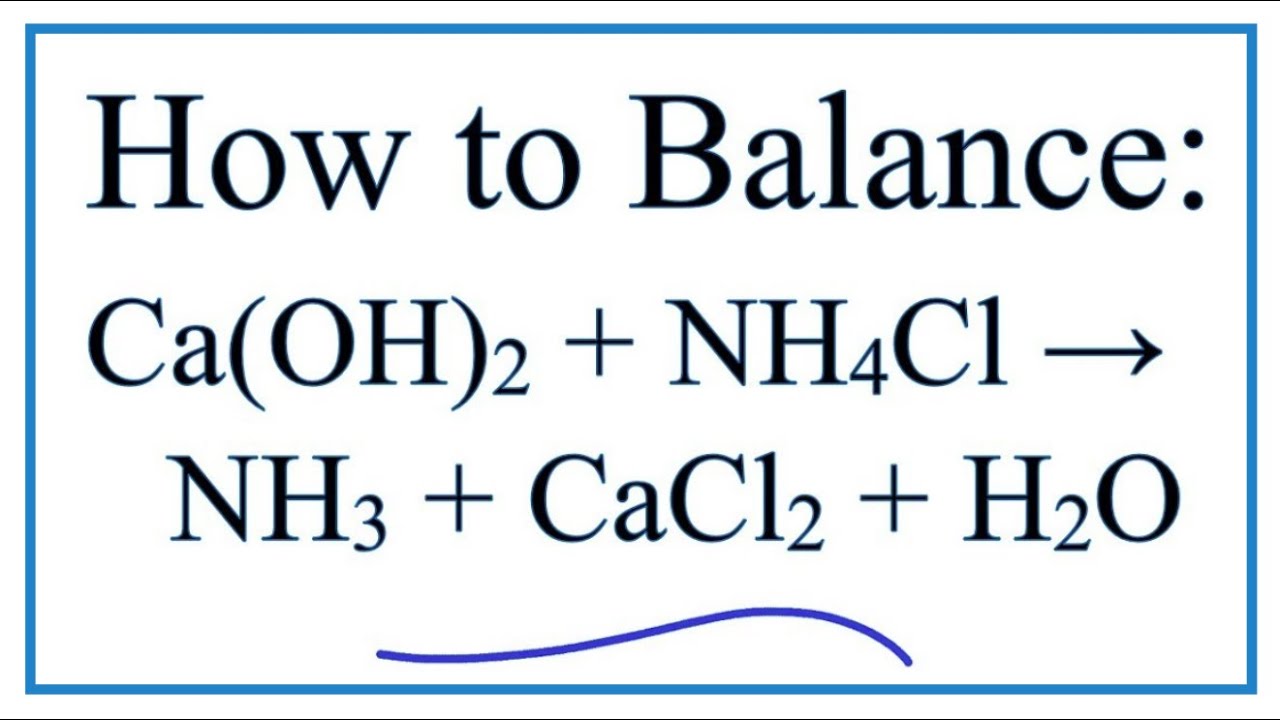
How To Balance Ca Oh 2 Nh4cl Nh3 Cacl2 H2o Youtube

Sigma Aldrich Ammonium Chloride Greater Than 99 5 Concentration 12125 02 9 53 49 Nh4cl Clear Glass Bottle 45zh26 A9434 1kg Grainger

Grade 11 Stoichiometry Is There Not A Need To Find Out The Limiting Reagent When We Calculate The Mass Of Ammonia R Homeworkhelp
Ammonium Chloride Nh4cl Pubchem
What Is The Gram Formula Mass Of Nh4cl Is How Is It Calculated Quora
What Is The Gram Formula Mass Of Nh4cl Is How Is It Calculated Quora
Comments
Post a Comment